Synthesis: cyclization of thiosemicarbazones
Many thiosemicarbazones derived from aldehydes are amenable to cyclization reactions affording five- or six-membered heterocycles with three heteroatoms, such as 1,2,4-triazoles, 1,2,4-triazole-3-thiones and 1,3,4-thiadiazolines. Thiosemicarbazones derived from aromatic nitriles can also undergo intermolecular condensation, giving rise to molecules with numerous potential donor atoms that can act as polydentate chelating ligands capable of forming polynuclear complexes. We are currently investigating thiosemicarbazones derived from 2‑cyanopyridine and 2-cyanopyrazine, studying in particular the influence of the substituents on the amide nitrogen atom on their susceptibility to cyclization in the presence of various metal ions and on the kind of heterocycle obtained.
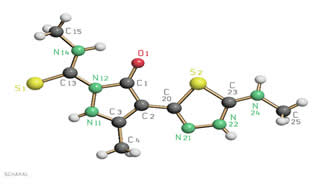
Pz134tda
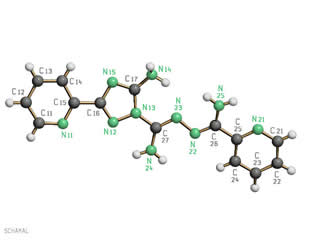
Am124taz
